Regarding the prevention of influenza, WHO’s latest recommendation of influenza vaccine strains in 2024-2025.
Foreword
Influenza virus can cause seasonal influenza, and may even cause a pandemic outbreak. Influenza virus is a negative-strand RNA virus with complex classification, numerous subtypes and easy mutation. At present, it is recognized that the best way to prevent influenza is vaccination. In order to ensure the effectiveness of the vaccine, the World Health Organization (WHO) updates the composition of the vaccine strain every year.
Changes of influenza vaccine strains in the northern hemisphere in 2024-2025
In order to keep the vaccine effective, it is necessary to update the components of influenza virus vaccine regularly. After analyzing the global influenza virus epidemiology, etiology and vaccine serology, WHO announced the recommendation of influenza vaccine components in the northern hemisphere from 2024 to 2025 on February 23, 2024.
?
Trivalent chicken embryo culture vaccine
an A/Victoria/4897/2022 (H1N1)pdm09-like virus
an A/Thailand/8/2022 (H3N2) -like virus
a B/Austria/1359417/2021 (B/Victoria lineage)-like virus
?
Trivalent cell culture or recombinant protein vaccine
an A/Wisconsin/67/2022 (H1N1)pdm09-like virus
an A/Massachusetts/18/2022 (H3N2)-like virus
a B/Austria/1359417/2021 (B/Victoria lineage)-like virus
?
Tetravalent chicken embryo and cell culture or recombinant protein vaccine
The following components are added on the basis of trivalent vaccine
a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus
Since March 2020, the naturally occurring B/Yamagata virus has not been confirmed. WHO thinks that it is not necessary to use B/Yamagata virus as a component of influenza vaccine, and it should be removed from the component of influenza vaccine. Where tetravalent vaccine is still used, the composition of B/Yamagata line is consistent with the previous recommendation.
Comparing the composition of influenza vaccine in the northern hemisphere from 2023 to 2024, it was found that the change of composition was mainly due to the difference of H3N2 virus strains:
Vaccine types
2023-2024
2024-2025
Trivalent chicken embryo culture vaccine
A/Darwin/9/2021 (H3N2)-like virus
A/Thailand/8/2022 (H3N2) -like virus
Trivalent cell culture or recombinant protein vaccine
A/Darwin/9/2021 (H3N2)-like virus
A/Massachusetts/18/2022 (H3N2)-like virus
Recombinant antigen is the key material for vaccine research.
In influenza virus-related vaccines, drugs and even diagnostic reagents, key target antigens have played an important role. In the following table, we have sorted out the information of HA, NA and NP proteins.
albumen
function
app; application
HA
(hemagglutinin)
The sialic acid receptor on the host cell membrane binds to help the virus envelope fuse with the host cell membrane; Coagulation
Basic research on influenza
Influenza vaccine research and development
Development of anti-hemagglutinin antibody
Study on virus detection reagent
NA
(neuraminidase)
Hydrolyze sialic acid receptor to promote the release of mature virus particles
Research and development of antiviral drugs
Basic research on influenza
Influenza vaccine research and development
Antibody development
Study on virus detection reagent
NP
(nuclear protein)
Combine with influenza genetic material and participate in virus gene replication, transcription and translation; Maintain the stability of virus gene
Research and development of antiviral drugs
Basic research on influenza
Antibody development
Diagnostic product development
The recombinant antigen of influenza vaccine strain can be used in all aspects of vaccine development, such as vaccine content detection, vaccine biological titer detection and toxicological experimental research. Using recombinant antigen for ELISA detection, we can analyze the levels of serum total antibody and neutralizing antibody after vaccination, and use recombinant protein as control to detect the vaccine content.
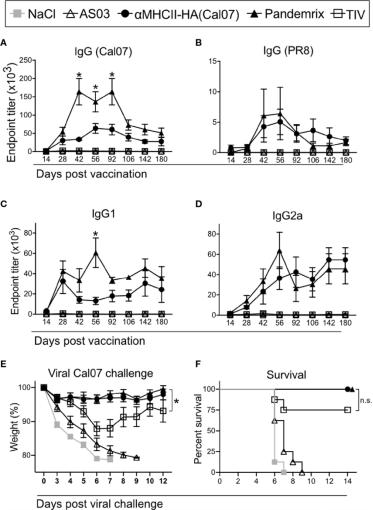
The results of detecting the serum antibody level of mice immunized with vaccine by recombinant influenza HA protein (image from Andersen, et al.)
Influenza vaccine strain-related antigens are available in stock.
Yiqiao Shenzhou continues to pay attention to the research progress of influenza vaccine, and has built a relatively complete influenza virus research reagent library. It has been online to recombine some proteins of HA, NA and NP of the northern hemisphere influenza vaccine strain in 2024-2025, and it is available from stock. Order it now!
Consultation is polite!
After checking the information correctly, you can get a 35 yuan Luckin Coffee voucher.
Scanning code to understand the recombinant antigens related to components of influenza vaccine strains from 2024 to 2025;
references
[1]https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2024-2025-northern-hemisphere-influenza-season
[2] Andersen, et al. Pandemic Preparedness Against Influenza: DNA Vaccine for Rapid Relief.[J]. Frontiers in Immunology.2021.747032.
[3] Bangaru S , et al. A Site of Vulnerability on the Influenza Virus Hemagglutinin Head Domain Trimer Interface[J]. Cell, 2019.
[4] Kim M, et al. Inhibition of infection virus internalization by (-)-Epigallocatechin-3-gallate. [J]. Antiviral Research, 2013,100 (2): 460-472. Continue to slide to see the next one.
Read the original text